Survival rate of childhood leukemia has improved considerably but the increased number of long-term survivors is now attended by a growing awareness that many will develop health conditions as a direct consequence of their treatments.
A first step for developing accurate strategy to reduce these long-term side effects (metabolic syndrome, cardiomyopathy, secondary tumors, osteonecrosis…) requires identification of their incidence and clinically detectable risk factors at a genetic level.
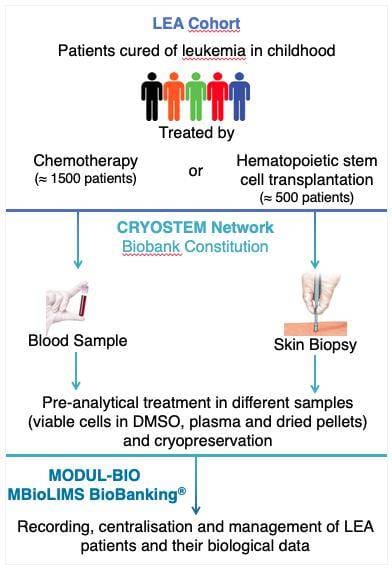
CRYO-LEA: creation of a new biobank in the field of leukemia
Modul-Bio has provided the solution MBioLIMS BioBanking® to manage and centralise patients and samples information to facilitate this biobank and further studies into genetic susceptibility.
LEA patients and their biological data are stored securely with users only permitted to view data that is relevant to them. Each 23 participating centres can work independently on their patients and samples data, and record the different samples collected.
Depending on the therapies a patient received to treat leukemia, the software adapts to manage the samples expected.
A supervising site is able to administer and analyse the data from all sites which provides a powerful centralised source of information that can be interrogated and reported on to provide any specific information as required.
Patient consent is held within the system in line with the new European privacy regulation GDPR, and the software will respond appropriately when consent is missing or withdrawn.
MBioLIMS BioBanking® ensures an auditable and automated sample life cycle history maintaining the custody and status of biological material as it is processed and researched.
The constitution of CRYO-LEA biological collection is a crucial step. Inclusions of LEA patients in Cryostem biobanks have begun during summer 2018.
“Modul-Bio ensures a major role by deploying a functional BIMS Biobank Information Management System, tailored to the biobank specificities, centralising a large number of samples and data, further used in genome-wide studies to identify genetic factors behind late side effects” says Dr Emilie Robert, Project leader at CRYO-LEA.