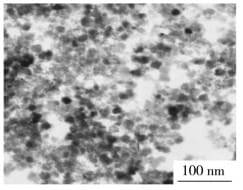
Cobalt iron oxide nanoparticles, the chemical formula of which is CoFe204, is a nanoparticle material composed of iron and cobalt elements. It has a high specific surface area and good magnetic properties, and is often used in catalysis, batteries, storage media and other fields.
And because magnetic nanoferrite particles have biosafety properties, they have important applications in biomedical fields such as:
They have become one of the current hot spots in nanomagnetic material research.
There are two methods for preparing cobalt iron oxide nanoparticles, physical method and chemical method.
Physical methods have major limitations. For example, the requirements for equipment are high, and the product is relatively easy to be oxidized. The properties of the product are not perfect, such as uneven particle distribution and low purity. Therefore, physical methods are generally not used.
Therefore, chemical methods are generally used to prepare cobalt iron oxide nanoparticles to obtain cobalt iron oxide nanoparticles with good quality and uniform morphology. At the same time, chemical methods are simple to operate and have lower production costs than physical methods.
Therefore, chemical methods are currently the most important method for preparing nanoscale cobalt ferrite. Currently, there are many methods for preparing magnetic nanoscale cobalt ferrite using chemical methods, such as precipitation methods, sol-gel method, hydrothermal method, electrospinning method, etc.
1. Precipitation method: The precipitation method refers to controlling appropriate conditions in a solution containing metal salts to allow metal ions to react with the added precipitant to form oxidized hydrates or insoluble compounds. The solvent-converted precipitate is then separated, dried, or thermally decomposed to obtain nanoparticles. This method is simple and easy to operate, and has become one of the commonly used methods for preparing nanoscale cobalt ferrite.
2. Sol-gel method: The reaction process of the sol-gel method can be divided into: (1) First, the precursor is converted into nanoparticles, then a colloidal suspension or sol is formed, and finally a gel network is formed. In experiments, some researchers used citric acid, ethyl orthosilicate and tetraethoxysilane as precursor solutions, combined with the metal nitrate gel method to prepare CoFe204-SiO2 composite nanoparticles.
3. Hydrothermal method: Hydrothermal method refers to a chemical reaction carried out in a sealed container at a certain temperature and pressure. Hydrothermal method includes hydrothermal method and solvothermal method. The dispersion media are different, one is water and the other are other solvents, and the reaction conditions are carried out under high temperature and pressure, among which the hydrothermal method is the most commonly used. The hydrothermal method can be used to synthesize CoFe204 nanoparticles, and time, temperature and surfactants have an impact on the size of CoFe204 nanoparticles.
4. Electrospinning method: Electrospinning method is the most common preparation technology for preparing nanofibers. Electrospinning method is simple to operate, has controllable process, and is cost-effective. Researchers have used electrospinning technology to prepare CoFe204 one-dimensional nanofibers and nanoribbons. The nanofibers and nanoribbons are composed of PVP and magnetic nanoparticles. The experiment obtained calcined nanoribbons at a temperature of 400 to 700°C. As the calcination temperature increases, the particle size in the one-dimensional structure gradually increases, and when the temperature reaches 900°C, the nanoribbons transform into nanofibers.
Cobalt iron oxide nanoparticles