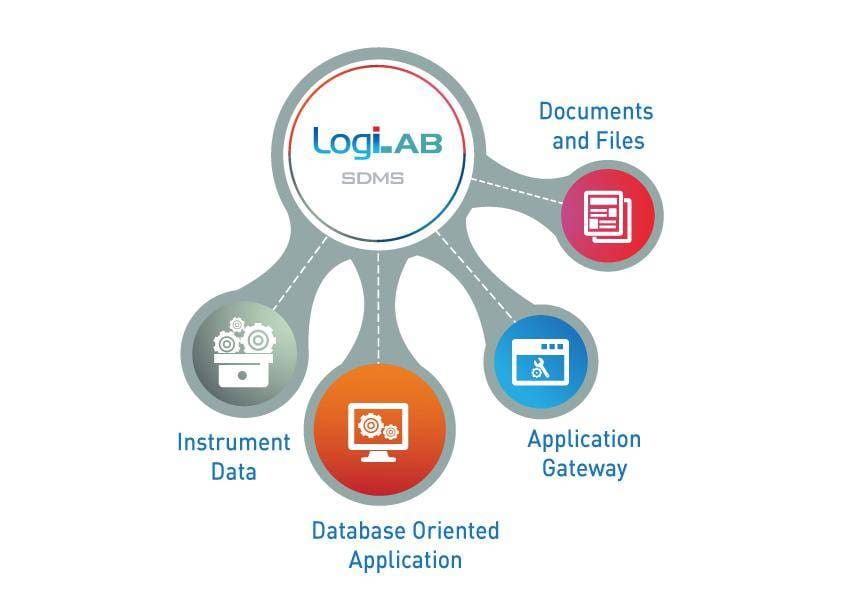
LogiLAB® SDMS is a data management system for laboratories in the regulated industry to achieve Data Integrity (ALCOA) and 21 CFR Part 11 compliance.
It can manage raw data along with metadata generated by laboratory instruments and other human-generated data (Excel, reports and documents); maintaining a single source of truth for GMP.
LogiLab sdms is a completely non-intrusive software that can be operated mostly on its own with minimal or no intervention required by users.
Its automated schedulers can capture data generated by any type of instrument independent of the manufacturer/ model.
Instrument data files generated by PC controlled instruments and data generated by standalone instruments can be captured via RS232 or TCP/IP ports.
LogiLab sdms can be deployed in quality control, process, R&D, contract research, pre-clinical and clinical research areas to achieve Data Integrity and 21 CFR Part 11 compliance.
Traditional methods of storing data files in a network drive or a file server are weak solutions when it comes to complying with 21 CFR Part 11, GLP, GMP and basics of Data Integrity based on ALCOA. LogiLab sdms provides a controlled environment with authorized access to data, audit trail & version control.
Central Repository for GMP Data:
– Capture & organize data in central repository
– Raw & metadata capture
– Independent of instrument& model
– PC based & standalone instruments
CFR Gateway Module- A Unique Solution for Data Integrity & 21 CFR Part 11
Benefits of CFR Gateway
– 21 CFR Part 11 & EudraLex Annex 11 compliance
– Do not replace old/ expensive instruments for compliance
– Single software for all your compliance needs
– No additional investment in individual instrument-based software for compliance
CFR Gateway
– Several Instruments do not have 21 CFR Part 11 capabilities
– Data integrity maintenance is also a challenge for instrument systems
– CFR Gateway is similar to virtual wrapper for non-compliant application
– Users Login through wrapper environment to access non-compliant software
– User Login/ Log-off is audit trailed (Controlled Access)
– Data generated/ modified by instrument is uploaded with version control
– Modification of data is always versioned & audit trailed
– No need to harden windows to protect data.
Technology & Architecture:
– Architecture: Client/ Server Application
– OS: Windows (XP, Vista, Win 7 & above)
– Database: MS-SQL Server
LogiLab sdms architecture
– Multi-site organisations (distributor architecture)
– Share data to a corporate location anywhere
– Authorizes personnel view data from multiple sites
– Data synch engine copy data to corporate sites (disaster avoidance/ recovery)