Targeted protein degradation (TPD) technology is a new technology that specifically recognizes the target protein and directly degrades the target protein using the inherent protein degradation pathway in the cell. At present, technologies such as PROTACs, molecular glues, degradation tags, lysosome-targeted chimeras, and autophagosome-binding compounds have been developed in the field of TPD, which greatly expands the range of degradable target proteins.
What exactly is TPD technology? While bringing good news to human health, what challenges and opportunities will TPD technology face?
Proteins are the basis of all living things, including human beings, and they are constantly being produced and dying in the body.
Most diseases are related to abnormal protein expression or activity, and selective elimination of pathogenic proteins will help cure diseases.
At present, the vast majority of drugs are protein inhibitors, which specifically bind to pathogenic proteins to inhibit their activity to obtain curative effects. However, the number of pathogenic proteins bound by available inhibitors is limited, less than 20% of the total number of potential pathogenic proteins, and 80% of the pathogenic proteins are undruggable targets.
In the 1970s and 1980s, Israeli scientists Aaron Sichanova, Avram Hershko and American scientist Irwin Ross discovered ubiquitin-mediated protein degradation after years of research. They were awarded the Nobel Prize in 2004.
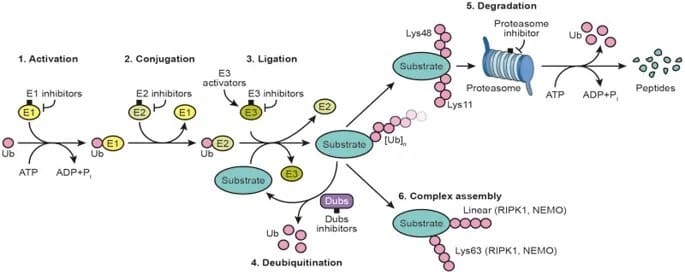
Ubiquitin is a polypeptide consisting of 76 amino acids that can form strong covalent bonds with proteins. Once the proteins are labeled with it, they will be sent to the “garbage disposal plant” in the cell, the proteasome for degradation.
TPD technology is a new technology that specifically recognizes the target protein and directly degrades the target protein by using the inherent protein degradation pathway in the cell. This is equivalent to finding disease-causing “targets” within cells and eradicating them.
The concept of targeted protein degradation was first proposed in 1999. In 2001, scholars proposed a more specific protein degradation targeting chimera (PROTAC) concept, and synthesized the first chimeric molecule targeting protein degradation. In 2013, Arvinas, the first listed company in the PAOTAC field, was born.
At present, technologies such as PROTACs, molecular glues, degradation tags, lysosome-targeted chimeras, and autophagosome-binding compounds have been developed in the field of TPD, which greatly expands the range of degradable target proteins.
Although the research results of the three Nobel Prize winners have laid the theoretical foundation of TPD, the research and development of new TPD drugs has only made substantial progress in recent years, and the technical challenges are self-evident. These challenges come from the fields of biology and medicinal chemistry, such as how to design, screen, and find molecules with good druggability.
The large molecular weight is considered by experts to be the first problem in the development of PROTAC drugs. According to the ‘Five Rules of Drug-like’, the molecular weight of small-molecule drugs should be below 500 Daltons, but the molecular weight of PROTACs is mostly above 700 Daltons, and the large molecular weight leads to low membrane permeability of the compounds. Most of the target proteins are present in cells and only when PROTAC molecules are small enough to pass through the cell membrane will the degradation activity be improved. Lipid solubility also affects the bioavailability of PROTAC drugs (the rate and degree of absorption of pharmaceutical preparations by the body). If a molecule can penetrate the cell membrane as much as possible, the bioavailability will be high. However, most of the PROTAC molecules are not very permeable. In order for the drug to exert its activity, a high dose must be given, which will solve the potential toxic and side effects and other problems.
For molecular glue drugs, most of the new drugs currently developed degrade proteins through CRBN, a component of E3 ubiquitin ligase, but even for proteins degraded through the CRBN pathway, the functions of many proteins are still unclear. In addition, molecular glues targeting most pathogenic targets are difficult to design.
When the research and development of TPD drugs is booming, the validation standards and guidelines for drugs are also urgently needed to be established. Many proteins have physiological functions, and it is difficult to predict which negative effects will be brought to the human body if the entire protein is removed. Secondly, the synthesis of TPD drugs such as PROTAC is complicated, the development speed is slow, the cost is high, and the synthesis steps need to be optimized.
However, it is undeniable that TPD has spawned unprecedented opportunities for new drug research and development. Given its better efficacy and wider application range than existing protein inhibitors, it is expected to fundamentally solve many diseases that were difficult to treat in the past, such as various tumors and neurodegenerative diseases. Many in the industry believe that its future market potential will be as high as trillions of dollars.