Monoclonal anti-drugs are a class of macromolecular protein drugs based on gamma-type immunoglobulin G (IgG). As one of the most important biopharmaceuticals, monoclonal antibody provides a new way to treat a variety of diseases, including cardiovascular disease, central nervous system disorders, autoimmune diseases, viral infections and cancer.
The rapid development of monoclonal antibody drugs and their increasing importance in the treatment of various diseases have put forward higher requirements for the development of drug resistance and drug safety.
In addition, the FDA issued the “Draft Guidelines for the Development of Biosimilar Products” in 2012, which gave the evaluation criteria for monoclonal antibody drugs and clearly pointed out that the macromolecular protein drugs for monoclonal antibodies should affect their effectiveness. Therefore, the qualitative and quantitative study of glycosylation modification of monoclonal antibody drugs is of great significance for drug development, improvement, and safe drug use.
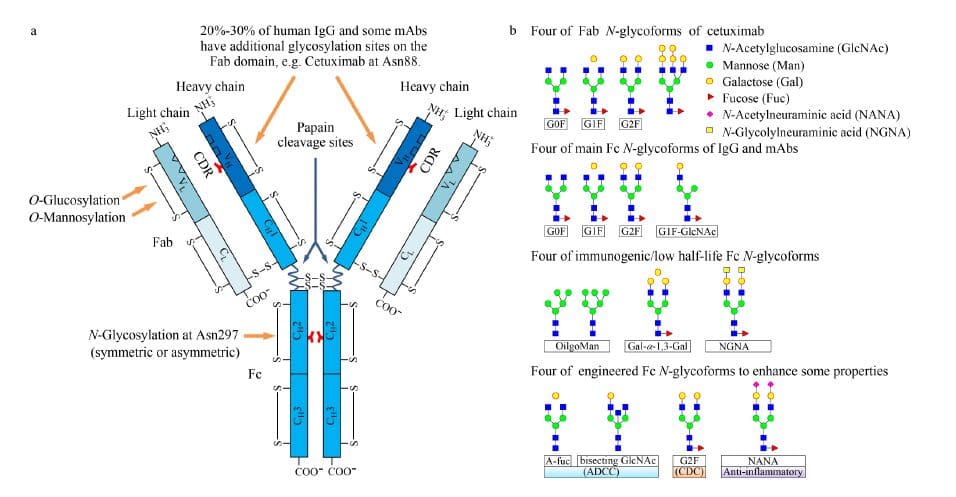
Some monoclonal antibodies have been found to have O-glycosylation modifications, such as the presence of a single mannose-modified O-glycosylation modification in monoclonal antibodies produced by Pichia pastoris.
A humanized monoclonal antibody has a single glucose-modified O-glycosylation modification produced by Chinese hamster ovary (CHO)1,2. The glycosylation modification of the monoclonal antibody is closely related to the production system of the monoclonal antibody, the selected cell line species, and the incubation process.
At present, the most commonly used cell incubation system is mammalian cells, including CHO cells, murine NSO cells, murine Sp2 / 0 cells and murine hybridoma cells. The glycosylation modification of monoclonal antibody produced by CHO cell line is the closest to the IgG’s glycosylation in the human, whereas glycosylation of monoclonal antibodies produced by murine NSO or Sp2 / 0 cells is relatively different3-5.
Numerous studies have shown that N-glycosylation of mAbs has a certain impact on stability, clearance, immunogenicity, antibody-dependent cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC)6, etc.
Qualitative and quantitative studies of glycosylation modifications of monoclonal antibodies have been carried out at various levels, including qualitative and quantitative characterization at intact protein levels, protein fragments, glycopeptides, and sugar chain levels.
ESI-MS coupled HPLC or size-exclusion chromatography (SEC) techniques are commonly used for the characterization of monoprotein-resistant glycosylation modifications at intact protein levels.
HPLC-MS has good separation ability for proteins, but it is often accompanied by strong adsorption and multi-peak formation. It can be improved by column material selection, mobile phase adjustment and elevated column temperature.
SEC is relatively simple and only needs to be separated at room temperature, but the resolution and efficiency are low and typically with problems like protein adsorption8,9. ESI-MS usually has high resolution and mass accuracy, and plays an important role in the characterization of monoclonal antibody glycosylation modification. It has been successfully used for the characterization of various monoclonal antibody glycosylation modifications.
HPLC method is frequently used for the qualitative and quantitative analysis of sugar chains. Because the UV absorption of sugar chains is weak, the sugar chains are often fluorescently labeled before analysis to improve the detection sensitivity.
2-Aminobenzamide (2-AB) and 2-amino-benzoic acid (2-AA) are the most commonly used fluorescent labeling reagents. The labeled sugar chain can be analyzed by techniques such as:
The released sugar chain can also be directly analyzed by high performance anion exchange chromatography combined with pulsed amperometric detection or thermal pyrolysis chromatography without fluorescent labeling, but the stability and reproducibility of the method are poor.
Protein glycosylation modification site identification and glycosylation modification at glycopeptide levels are typically performed by ESI-MS technology or MALDI-TOF MS technology, and ESI-MS is the most powerful tool for glycopeptide characterization.
A large amount of sugar chain information can be obtained by MS/MS spectrum, and the glycosylation site identification and sugar chain composition determination can be completed simply and efficiently by glycopeptide analysis software.
The complexity of glycosylation modification itself and the difficulty of detecting glycosylation modification have brought enormous challenges to related research. Although quantitative studies of glycosylation modifications of monoclonal antibodies have been developed at various levels and are rapidly evolving, there are still some problems, such as recovery and reproducibility of glycopeptide enrichment, throughput of sample processing, method sensitivity.
The simplification of the data analysis process and the establishment of the standardization process are subject to the development of new methods and techniques by analytical chemists, providing efficient and reliable analytical tools for the study of monoclonal antibodies.
Glycosylation Analysis