If your eye cream remains stable and usable even after sitting on a shelf for two years, you might be tempted to praise its good quality. However, can you really ignore the safety implications behind that long-lasting shelf life? From preserved lotions to stretch-mark creams, many cosmetics use complicated preservation strategies to maintain shelf life and effectiveness. Unfortunately, complicated preservation systems can also mean complex chemical safety considerations.
The term “formaldehyde” is largely absent from cosmetic ingredient lists these days. Despite this, you will still find it showing up time and time again in safety assessments, regulatory reviews, and product recalls. More often than not, it is not from the direct addition of formaldehyde but rather the slow reaction of formaldehyde-releasing preservatives (FRPs) or degradation of cosmetic ingredients in complex formulations. Formaldehyde management has become less about avoiding the use of certain ingredients as formulated brands and more about accurately identifying it during product testing.
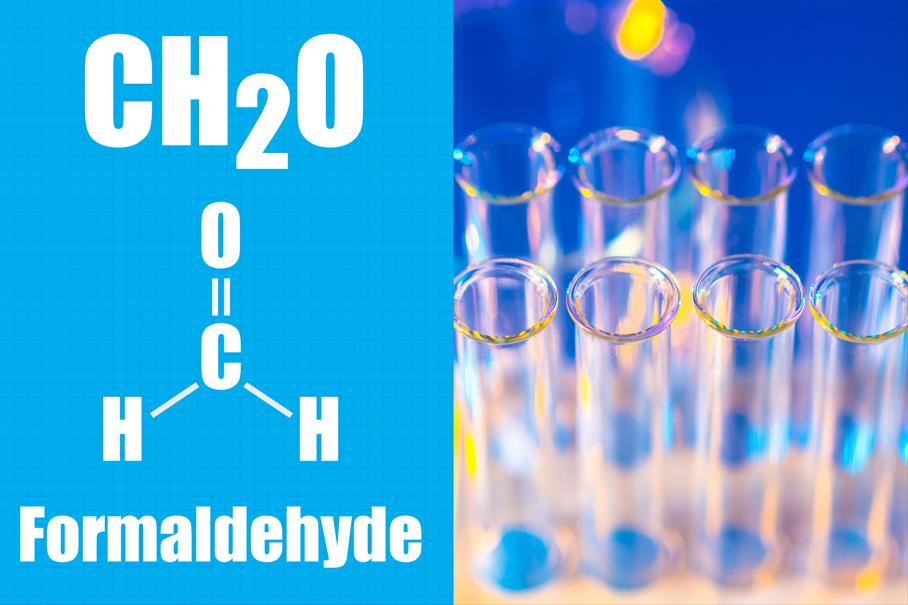
Formaldehyde is a small molecule with potent antimicrobial properties. Its size and chemical reactivity also contribute to its well-documented toxicological profile. Formaldehyde can readily react with other compounds in the body, which is why it has been linked to skin sensitization, irritation to the eyes and respiratory system, and carcinogenicity (at sufficient doses/exposure levels). Use of formaldehyde in cosmetics presents a unique exposure route in that it typically involves repeated long-term exposure on broken or damaged skin. Safety evaluation is less about “getting burned” and more about low levels of exposure over time.
Although direct use of formaldehyde as a preservative has been restricted in several markets, the chemistry did not go away. Instead, it found new life with formaldehyde-releasing preservatives, or FRPs. Formulated to slowly release small amounts of formaldehyde in water, these preservatives were able to provide extended antimicrobial efficacy at trace concentrations. From a preservative perspective, these ingredients were ideal—long-lasting protection and well established safety profiles. From a formaldehyde standpoint, not so much. When absorbed through the skin, it does not matter if formaldehyde is “free” or “released.”
To make matters worse, release rates of FRPs are not constant. Reaction kinetics can be affected by many formulation variables, including pH, storage temperature, exposure to free amines or other cosmetic ingredients, and even packaging. Simply increasing storage temperature by 10 °C can significantly increase the free formaldehyde concentrations observed. Two cosmetic products with the same preservative at the same claimed concentration could have extremely diverse free formaldehyde concentrations after stability testing. It starts off as a formulating challenge and quickly becomes a regulatory headache.
Formaldehyde can still be detected in cosmetic formulations that contain ZERO FRPs. Ingredient degradation from heat or light exposure, trace impurities from manufacturing, and even side reactions from nitrogen containing ingredients reacting with oxidizing agents can generate formaldehyde in cosmetic products. In these cases, formaldehyde was likely never an ingredient decision but rather a side effect of complex formulation chemistry. Sounds pretty tricky to claim your product is “formaldehyde-free,” doesn’t it? Claiming your product does not contain formaldehyde donors is one thing, but guaranteeing there is no detectable formaldehyde in your finished product is something entirely different.
Identifying formaldehyde in cosmetics is no easy feat. For one, it is volatile and highly reactive. Secondly, it is usually present at trace levels and is often found within complex matrices that contain surfactants, oils, polymers, and fragrance. Most methods for detecting formaldehyde will require you to first convert it to another stable derivative. Standard practice involves separation of the formaldehyde from your product matrix via HPLC or GC and analysis via a mass spectrometer. If FRPs are used in the formulation, you may even need to test multiple time points using stability or stress studies to determine release kinetics. Identifying formaldehyde in cosmetic products is no longer just an analytical question—how much formaldehyde could your consumer be exposed to over time?
Regulatory agencies and large retailers are catching on. Formaldehyde and formaldehyde donors are subject to strict limits, labeling requirements, and/or testing data submissions in many markets around the world. Claims of “fragrance-free,” “sensitive skin,” and even “safe” will require analytical data if your product contains FRPs. Especially when safety becomes the selling point, you can bet your competitors supplier specifications will not be enough to satisfy regulatory agencies, and safety assurances will become your responsibility.
The stakes are high at little levels, of course. Unknown formaldehyde detections can delay product shipments, force late-stage reformulations, and potentially harm your brand in a highly sensitized consumer market. Safety issues that began at the parts-per-million level can quickly become public relations disasters.
That is where Alfa Chemistry can help. Our formaldehyde in cosmetics testing service is specifically designed to identify formaldehyde and formaldehyde-releasing preservatives in cosmetic products. Using advanced chromatographic methods, we can separate formaldehyde from your product matrix and accurately quantify even at trace levels. In addition to simply identifying total concentrations, we can also assist with identifying possible sources, determining release rates from donors, and assisting with international safety assessments. Don’t let formaldehyde become the unknown in your safety and stability assessments.
Formaldehyde Analysis for Cosmetics